- President Droupadi Murmu has announced ‘NexCAR19’, India’s first CAR T-cell therapy developed in-house for cancer treatment.
What is CAR-T Cell Therapy?
| What is it? | The term CAR-T cell therapy refers to chimeric antigen receptor T cell treatment.It is a sort of cancer immunotherapy in which the patient’s own T cells are genetically engineered in a laboratory to improve their ability to detect and destroy cancer cells. |
| How does it work? | T cells are white blood cells that identify and combat disease and infection.Each T cell contains a receptor that can recognise antigens.Cancer cells may include antigens that the immune system may not recognise as aberrant, allowing cancer to elude immune detection.CAR-T cells are genetically created in the lab to express a novel receptor capable of binding to and killing cancer cells. |
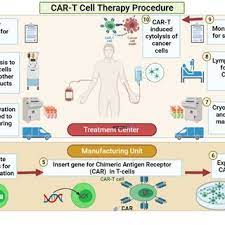
| Therapy Process | The procedure consists of multiple steps, including:1. T Cell Collection: Blood is collected from the patient’s arm and separated using an apheresis machine.2. Engineering T Cells: In a laboratory, T cells are transformed by inserting a produced CAR and allowed to replicate and expand.3. Infusing CAR-T Cells: Once the CAR-T cells have been manufactured, they are injected back into the patient’s arm.Chemotherapy may be used prior to CAR-T cell infusion to improve treatment efficacy.The procedure might take place in an outpatient infusion centre or in a hospital. |
| Cancers Treated | CAR-T cell therapy is beneficial in treating certain types of cancer, particularly when other treatments fail.It is now FDA authorised for the treatment of haematological malignancies such as leukaemia, lymphoma, and multiple myeloma. |
NexCAR19: India’s Self-Developed CAR-T Therapy
- NexCAR19 is intended to target cancer cells that express the CD19 protein, a cancer cell marker, thereby improving therapeutic accuracy.
- The Tata Memorial Centre and IIT Bombay collaborated in its development.
- Initially approved for patients aged 15 and older with B-cell lymphomas who did not respond to traditional therapies, resulting in relapse or recurrence.
Effectiveness and Unique Features
- Approximately 70% of patients react to NexCAR19 treatment, and some achieve complete remission.
- Lower drug-related toxicities, such as decreased neurotoxicity and Cytokine Release Syndrome (CRS), have been shown in laboratory and animal investigations.
- Trials for paediatric patients are under conducted at Tata Memorial Hospital, assuring wider applicability.
Availability and affordability
- ImmunoACT is in the process of acquiring licences and forming partnerships with hospitals in numerous locations, including Tata Memorial, Nanavati, Fortis, and Jaslok.
- ImmunoACT, which is initially priced at Rs 30-40 lakh, seeks to reduce the cost to Rs 10-20 lakh over time, making the therapy more accessible.
Source: https://pib.gov.in/PressReleasePage.aspx?PRID=2017169#:~:text=NexCAR19%20CAR%2DT%20therapy%20is,advanced%20cell%20and%20gene%20therapy.